▼: AIF : ANT : apoptotic appearance : apoptosomes : apoptotic bodies : Bcl-2 : Bcl-2 family : caspase cascade סּ caspases : cytochrome C : death domain : death receptor pathway : DISC : Endonuclease G : FADD : FAS gene : homeostasis and apoptosis : MBR : mitochondrial pathway : necrosis : PBR : porin : proteolytic cascade : PT pore : TNF-R : tumor suppressors : Smac/DIABLO : VDAC : ▼
Tumor suppressor proteins such as p53 act as cell-cycle repressors and/or promoters of apoptosis through:
1. interruption of cell cycle, preventing cell division,
2. halting the cell cycle if DNA damage is not yet repaired,
3. inducing apoptosis if DNA damage cannot be repaired,
4. promoting cell adhesion and contact inhibition, which prevent invasion and metastasis.
 Both the death receptor pathway and the mitochondrial pathway lead to the activation of an initiator caspase, which initiates a proteolytic cascade, ultimately leading to apoptotic cell death.
Both the death receptor pathway and the mitochondrial pathway lead to the activation of an initiator caspase, which initiates a proteolytic cascade, ultimately leading to apoptotic cell death.Necrosis results from impairment of the cell’s ability to maintain homeostasis due to breakdown of the plasma membrane. This leads to influx of water as pumps fail and osmotic gradients reverse. Ultimately organelles (especially mitochondria) swell and hydrolytic, lysosomal enzymes are released into the cytosol, leading to cellular swelling and rupture (lysis). So, in vivo, necrotic cell death is often associated with extensive tissue damage resulting in an intense inflammatory response.
Apoptosis, by contrast, occurs under normal physiological conditions when the cells respond to signals as active participant in their own deaths. Apoptosis is a part of normal cell turnover and tissue homeostasis. It plays a significant role in embryogenesis, induction and maintenance of immune tolerance, development of the nervous system, and atrophy of endocrine-dependent tissue.
[] Artist's impression apoptosis []
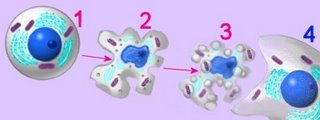 Cells undergoing apoptosis show characteristic morphological and biochemical features, which include chromosomal changes: chromatin aggregation, nuclear and cytoplasmic condensation (2), and partition of cytoplasm and nucleus into apoptotic bodies (apoptosomes). These are membrane bound-vesicles that contain ribosomes and intact mitochondria and nuclear material (3). Apoptotic bodies are rapidly recognized in vivo and and are phagocytozed by either macrophages or adjacent epithelial cells (4), without inflammatory response. In vitro, apoptotic bodies and remaining cell fragments ultimately swell and lyse (“secondary necrosis"). [more] Џ apoptosis animations Џ
Cells undergoing apoptosis show characteristic morphological and biochemical features, which include chromosomal changes: chromatin aggregation, nuclear and cytoplasmic condensation (2), and partition of cytoplasm and nucleus into apoptotic bodies (apoptosomes). These are membrane bound-vesicles that contain ribosomes and intact mitochondria and nuclear material (3). Apoptotic bodies are rapidly recognized in vivo and and are phagocytozed by either macrophages or adjacent epithelial cells (4), without inflammatory response. In vitro, apoptotic bodies and remaining cell fragments ultimately swell and lyse (“secondary necrosis"). [more] Џ apoptosis animations ЏTables Apoptosis vs Necrosis Apoptosis
Death receptor pathway:
Death receptors include the TNF-R (tumour necrosis factor receptors) and CD95 (Apo-1 or Fas) receptor families. Ligands for these death receptors are termed TNF-α and CD-95L (FasL), respectively. TNF-α is a highly cytotoxic molecule and the TNF-R1 receptor is widespread, with the result that TNFα has a low tolerated dose. Several members of the TNF-R1 family share a homologous region known as the death domain (DD), which is a protein-protein interaction domain that binds to adaptor proteins such as the adaptor protein FADD (Fas-Associated Death Domain). The Death-Inducing Signalling Complex (DISC) comprises death receptor ligands, death receptors and adaptor proteins such as FADD. Activation of death receptors by binding of ligands such as CD-95L (FasL) and TNF-α leads to caspase-8 activity.
Mitochondrial pathway:
Mitochondria play a central role in apoptosis and display an increase in mitochondrial membrane permeability during apoptosis. Mitochondria participate in apoptosis, and may be necessary for induction of apoptosis by apoptotic stimuli such as DNA damage. Pro-apoptotic and anti-apoptotic members of the Bcl-2 family are believed to regulate the release, through the mitochondrial PT pore, of pro-apoptotic substances such as AIF, Endonuclease G, Smac/DIABLO and cytochrome C. Mitochondrial efflux of cytochrome-c drives generation of the apoptosome (apoptotic body) in the cytoplasm. This in turn leads to caspase-9 activity.
Bcl-2 family: Members of the Bcl-2 subfamily (Bcl-2, Bcl-xL, Bcl-w) are localized on the outer mitochondrial membrane and show anti-apoptotic activity. They possess all four BH (Bcl-2 Homology) domains (BH1 to BH4). (Anti-apoptotic members of the Bcl-2 family have all four BH domains, while pro-apoptotic members have less BH domains.↓) Bax subfamily (Bax, Bak, BAD) : Bax is Bcl-2 Associated X protein, a pro-apoptotic member of the Bcl-2 family, which contains BH1 to BH3. BH3 only subfamily: Bid or tBid is (truncated) BH3-Interacting Domain death agonist, is a soluble, pro-apoptotic member of the Bcl-2 family, which contains only the BH3 domain.
Pro-apoptotic proteins : Bad, Bax, Bid, Bak, Bik, Bim, Bmf, Bok, Puma.
Anti-apoptotic proteins : Bcl-2, Bcl-XL, Mcl-1, Bcl-w
Released through the Mitochondrial Permeability Transition Pore (PT pore) are:
1. Apoptosis Inducing Factor (AIF) is a flavoprotein that is translocated to the nucleus where it causes DNA fragmentation into fragments of about 50kb.
2. Endonuclease G (Endo-G) is translocated to the nucleus, where it degrades single stranded DNA.
3. Smac/DIABLO is "Second Mitochondrial Activator of Caspases/Direct IAP Binding protein with Low pI", which inhibits IAP activity, and is therefore pro-apoptotic.
4. Cytochrome c is an essential component for the transportation of electrons during mitochondrial oxidative phosporylation. When released from the mitochondria, cytochrome c drives the formation of the apoptotic body (apoptosome).[s, Refs]
The PT pore is constituted of ANT, PBR (MBR), and VDAC (porin), and the mitochondrail membrane porosity is modified by Bcl-2 regulators.
Tables Apoptosis vs Necrosis Apoptosis Џ beautiful Flash 8 animation - Inner Life of the Cell : and Interpretation: Inner Life of the Cell Џ
• A • adhesion • C • cell membranes • cellular adhesion molecules • cellular signal transduction • centrioles • chemotaxis • chloroplast • cilia & flagella • communication • concentration gradients • cytokine receptors • cytoplasm • cytoskeleton • E • energy transducers • endoplasmic reticulum • endosomes • exosome • F • flagella & cilia • G • Golgi apparatus • GPCRs • H • hormones • I • ion channels • L • lysosome • M • meiosis • microtubules • mitosis • mitochondrion • N • Nitric Oxide • neurotransmission • neuronal interconnections • nuclear membrane • nuclear pore • P • pinocytosis • proteasome • protein degradation • pumps • R • receptor proteins • receptor-mediated endocytosis • S • second messengers • signaling gradients • signal transduction • spindle • structure • T • transport • two-component systems • U • ubiquitin • V • vacuole • vesicle •
Џ Quicktime video apoptosis : Apoptosis and Caspase :
Genome Biology Full text DNA-damage signaling and apoptosis: "Cytochrome c binds the apoptosis-activating factor 1 (Apaf1) protein, leading to oligomerization of Apaf1 and caspase 9 into a large 'apoptasome', which then initiates a cascade of caspase activation. Although some non-caspase targets of caspase activation are known, the consequences of proteolysis of these targets are not well understood. Similarly, the events upstream of activation of the caspase cascade in response to DNA damage are not well known; in particular, it is not clear what regulates the decision to undergo apoptosis or to arrest cell proliferation and repair the damage."
apoptosis: Bcl-2 proteins
Apoptosis - Bcl-2 proteins: "The bcl-2 proteins are a family of proteins involved in the response to apoptosis. Some of these proteins (such as bcl-2 and bcl-XL) are anti-apoptotic, while others (such as Bad or Bax) are pro-apoptotic ↑. The sensitivity of cells to apoptotic stimuli can depend on the balance of pro- and anti-apoptotic bcl-2 proteins. When there is an excess of pro-apoptotic proteins the cells are more sensitive to apoptosis, when there is an excess of anti-apoptotic proteins the cells will tend to be less sensitive."
Џ apoptosis animations : Kimball's Apoptosis Page : Apoptosis - Website : Apoptosis Website : Wikipedia on apoptosis : pathology : Cell Suicide in Health and Disease : Google apoptosis Џ animation - zeiosis Џ Apoptosis, Bcl2, Mitochondria~click on Q for animation Џ Apoptosis~time-lapse movie : art~apoptosome formation : art~inactivation of DNA repair enzymes : Cell Death Pathways - diagram
▲: AIF : ANT : apoptotic appearance : apoptosomes : apoptotic bodies : Bax : Bcl-2 : Bcl-2 family : caspase cascade סּ caspases סּ cellular stress response : cytochrome C סּ death of cells : death domain : death receptor pathway : DISC : Endonuclease G : FADD : Fas gene ○ FAS gene : homeostasis and apoptosis : MBR : mitochondrial pathway סּ mitochondria : necrosis : PBR : porin : proteolytic cascade : PT pore : TNF-R ○ TNF-R : tumor suppressors ¤ tumor suppressors : Smac/DIABLO : VDAC : ▲
▲ Top ▲
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.