▼: actin : adhesion : cadherins : catenins : desmin : desmoplakin : desmosomes : intermediate filaments : intermediate-filament associated proteins : intermediate filament structure : heterodimers, homodimers : keratins : keratin diversity : lamins : lattice : macula adherens : microfilaments : microtubules : receptor control : vinculin :▼
Cellular cytoplasm is dominated by the viscoelastic network of the cytoskeletal lattice, comprising microfilaments (actin filaments and contractile actomyosin filaments), microtubules, and intermediate filaments. Cytoskeletons exhibit 'tensegrity' – short for tensional integrity. They balance compression with tension, and yield to forces without breaking. The cytoskeletal lattice is directly responsible for determining cell shape, generating mechanical forces, resisting externally imposed forces, and transducing extracellular biochemical and mechanical stimuli to the cytoplasm. Cytoskeletal dynamics enable the remodeling that is necessary for cell migration and chemotaxis that underlies tissue development, the inflammatory response, and tumor invasion and metastasis.
Cell shape also can determine cellular fate independently of and in addition to the formation of adhesive contacts with surrounding cells or the extracellular matrix (ECM). Utilizing the cytoskeleton as a vehicle for signal transduction, mechanical forces play a central role in driving a wide variety of physiological events including tissue remodelling, cutaneous mechanosensation, and auditory processing by hair cells (sem image - inner ear stereocilia). Human disease may arise from excessive input to the mechanotransducing cell, genetic lesions in the mechanotransduction apparatus, or alterations of cell or tissue mechanics.[s]
: must see gallery of fluorescence microscopy : cytoskeletal components : intermediate filaments - keratins (purple) lamins (green) : microtubules filaments : fluor-micro fibroblast cytoskeleton : fluor-micrograph cytoskeleton tubulin(green) F-actin(red) paxillin(blue) : F-actin plant cytoskeleton : fluorescence micrographs gallery : spindle : fluo-micro mitosis :
Џ beautiful Flash 8 animation - Inner Life of the Cell, which shows interactions between adhesion-signaling molecules and the cytoskeleton, the scaffolding lattices and conveyor belt mechanisms, and assembly/disassembly of actin and tubulin, and Interpretation: Inner Life of the Cell Џ
Microfilaments are 3-6 nm in diameter, and are composed mostly of the contractile protein actin – the most abundant cellular protein. Microfilaments are responsible for the cellular movements of gliding, contraction, and cytokinesis (division of the cytoplasm). The association of micofilaments of actin with the protein myosin is central to muscle contraction. image_filamentous actin microtubules nuclei : image_filamentous actin & microtubules : image_microtubules nuclei endothelial tc : image_filamentous actin microtubules nuclei fibroblast mouse : image_tubulin microtubules : image - cytoskeleton : image_cytoskeleton : diagram - mechanism of ciliary motility :
Intermediate filaments (IFs) are about 10 nm diameter. They are relatively stable molecules that provide tensile strength for the cell and connect adjacent cells through desmosomes (macula adherens). Each intermediate filament monomer comprises a central alpha helical rod domain capped with globular amino (head) and carboxyl (tail) terminals. Intermediate filaments are constructed of homodimers or heterodimers that form staggered tetramers aligned head-tail. Spacer sequences in the coiled coil, sequences in the diverse N- or C-terminal domains, or both most likely are responsible for determining whether particular intermediate filament proteins will assemble into heteropolymers or homopolymers.
Two monomer units form a coiled-coil dimer that self-associates to form the staggered tetramer in an anti-parallel arrangement. This is the analogous soluble subunit for the globular actin monomer and the tubulin heterodimer. Tetramer units pack together laterally, forming a sheet of eight parallel protofilaments supercoiled into a tight bundle. Each tightly coiled intermediate filament reveals 32 individual alpha-helical peptides in cross-section, rendering the filament supple but quite difficult to break, accounting for structural rigidity. Some classes of IFPs are highly dynamic structures with a significant rate of turnover in many cell types.
Cells possess five types of intermediate filament:
1. Type I – acidic keratin heterodimer of epithelial cells
2. Type II– basic keratin heterodimer of epithelial cells
3. Type III– distributed in a number of cell types, including–
a. vimentin in fibroblasts, endothelial cells, and leukocytes - vimentin is frequently associated with microtubules, so the network of vimentin filaments parallels the microtubule network
b. desmin in muscle
c. glial fibrillary acidic factor/protein (GFAP) in astrocytes and other types of glia, and
d. peripherin in peripheral nerve fibers.
4. Type IV neurofilament –
a. H (heavy), M (medium) and L (low)
b. "internexin"
c. nonstandard IV's are found in lens fibers of the eye (filensin and phakinin).
5. Type V – lamins have a nuclear signal sequence and form a filamentous support inside the inner nuclear membrane. Of the three nuclear lamins, two are alternatively spliced products encoded by a single gene, while the third lamin is encoded by a separate gene. Nuclear lamins form a fibrous network that supports the nuclear membrane. Lamins have a very long rod domain and carry a nuclear transport signal, they are located in the nucleus just beneath the nuclear envelope so they are vital to the re-assembly of the nuclear envelope after cell division. Lamins are phosphorylated at the end of prophase and this causes them to disassemble simultaneous with dissolution of the nuclear envelope. After cell division, they are dephosphorylated just before the nuclei of the daughter cells form and lamin filaments reassemble around each set of chromosomes. They are continuous except for a break at the sites of nuclear pore complexes. Lamins were probably the first intermediate filaments to evolve. It is believed that nuclear lamins are the evolutionary ancestor of cytoplasmic intermediate filaments, which evolved through duplication and translocation of the gene product to the cytoplasm.
Controlled by the serine/threonine cyclin-dependent protein kinase, cdc2 kinase (cdk1), filaments of vimentin, desmin, and lamins disassemble prior to or early in mitosis then reassemble after cell division. Phosphorylation of serine residues in the N-terminal domain of lamin A and vimentin by cdc2 kinase induces the disassembly of intact filaments and prevents reassembly. Phosphorylation by cdc2 and MEK are required for the disassembly of the Golgi apparatus prior to post-mitotic partitioning into two daughter cells (cytokinesis). Regulation of cyclin-dependent kinase activity is a complex process in that activation of catalytic subunits requires binding with the subset of regulatory subunits called cyclins. Other regula-
tory proteins activate or inhibit CDK by phosphorylation, dephosphorylation or binding to
CDK.
Keratins are most diverse IFPs, and the human genome contains at least 49 different keratin genes, all encoding proteins that combine into intermediate filaments of the epithelial cell cytoskeleton. Another 16 non-keratin genes code for similar intermediate filaments in other tissues.
Intermediate filament associated proteins improve internal stability by forming cross-linked binding, or they may bind the filaments to other structures.
a. Plectin cross links with microtubules
b. Lamin receptor B binds to the inner nuclear membrane
c. Ankyryn binds actin to intermediate filaments at the base of cells
d. Desmoplakin binds intermediate filaments at the desmosome
diagram . desmosome : tem_desmosome : diagram . tight junction : diagram . gap junction : detail : micrograph keratin intermediate filaments :
Cell adhesion molecules (CAMs) interact with cytoskeletal components to control cell adhesion, cellular morphogenesis, cellular migration, and cell signaling. Rho GTPases play a role in reorganization of the actin cytoskeleton, cell movement, chemotaxis, and axonal guidance.
In epithelial tissues, keratin intermediate filaments form junctions holding cells together (desmosomes), or attaching cells to the matrix (hemidesmosomes). In muscle cells, the intermediate filaments that form the desmosome are termed "desmins". In desmosomes two plaques desmoplakin and other proteins on adjacent cells are connected by cadherin molecules. The intermediate filaments loop into the plaques spreading out into the cytoplasm.[s]
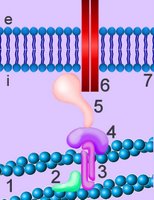 Cadherins are developmentally regulated, calcium-dependent homophilic cell-cell adhesion proteins (right - click to enlarge). The classic cadherins are defined by a conserved intracellular (i) domain which mediates interactions with cytoplasmic proteins termed catenins: α- and β-catenin. β-catenin (5) binds to both the C-terminus of the cadherin intracellular domain (6) and the N-terminus of α-catenin (4). α-catenin binds to a number of proteins involved in actin binding, bundling and polymerisation, as well as binding directly to F-actin of the cytoskeleton, here through α-actin (3) in association with vinculin (2). Absence of α- or β-catenin results in defective cell adhesion and failure of cadherin-catenin complexes to associate with the actin cytoskeleton.
Cadherins are developmentally regulated, calcium-dependent homophilic cell-cell adhesion proteins (right - click to enlarge). The classic cadherins are defined by a conserved intracellular (i) domain which mediates interactions with cytoplasmic proteins termed catenins: α- and β-catenin. β-catenin (5) binds to both the C-terminus of the cadherin intracellular domain (6) and the N-terminus of α-catenin (4). α-catenin binds to a number of proteins involved in actin binding, bundling and polymerisation, as well as binding directly to F-actin of the cytoskeleton, here through α-actin (3) in association with vinculin (2). Absence of α- or β-catenin results in defective cell adhesion and failure of cadherin-catenin complexes to associate with the actin cytoskeleton.Harvard video of actin-myosin action.
▲: actin : adhesion ~ adhesion items ~ adhesion molecules : cadherins ~ cadherins : catenins : desmin : desmoplakin : desmosomes : homodimers, heterodimers : intermediate filaments : intermediate-filament associated proteins : intermediate filament structure : keratins : ketratin diversity : lamins : lattice : macula adherens : microfilaments : microtubules : receptor control : vinculin :▲
• A • adhesion • C • cell membranes • cellular adhesion molecules • cellular signal transduction • centrioles • chemotaxis • chloroplast • cilia • communication • concentration gradients • cytokine receptors • cytoplasm • cytoskeleton • E • energy transducers • endoplasmic reticulum • endosomes • exosome • G • Golgi apparatus • GPCRs • H • hormones • I • ion channels • L • lysosome • M • meiosis • microtubules • mitosis • mitochondrion • N • Nitric Oxide • neurotransmission • neuronal interconnections • nuclear membrane • nuclear pore • P • pinocytosis • proteasome • pumps • R •receptor proteins • receptor-mediated endocytosis • S • second messengers • signaling gradients • signal transduction • spindle • structure • T • transport • two-component systems • V • vacuole • vesicle •
▲ Top ▲
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.