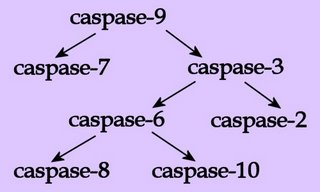
 At least 14 caspase isoforms have now been identified, and the isoforms are broadly categorised into initiators, effectors and inflammatory caspases. Initiator caspases such as caspase-8 and 9 cleave and activate effector caspases such as caspase-3. Effector caspases cleave specific proteins that ultimately leads to cell death by apoptosis. Caspase activity leads to a proteolytic cascade in which one caspase can activate other caspases, thus amplifying the apoptotic signalling pathway.
At least 14 caspase isoforms have now been identified, and the isoforms are broadly categorised into initiators, effectors and inflammatory caspases. Initiator caspases such as caspase-8 and 9 cleave and activate effector caspases such as caspase-3. Effector caspases cleave specific proteins that ultimately leads to cell death by apoptosis. Caspase activity leads to a proteolytic cascade in which one caspase can activate other caspases, thus amplifying the apoptotic signalling pathway.Cleavage of proteins by caspases can either activate them (other caspases, ICAD), or they can deactivate them (PKB/Akt, Raf-1, PARP-1). In general, proteins that promote apoptosis are activated, and proteins that promote survival are inactivated.
Tables Apoptosis vs Necrosis Apoptosis
• A • adhesion • C • cell membranes • cellular adhesion molecules • cellular signal transduction • centrioles • chemotaxis • chloroplast • cilia & flagella • communication • concentration gradients • cytokine receptors • cytoplasm • cytoskeleton • E • energy transducers • endoplasmic reticulum • endosomes • exosome • F • flagella & cilia • G • Golgi apparatus • GPCRs • H • hormones • I • ion channels • L • lysosome • M • meiosis • microtubules • mitosis • mitochondrion • N • Nitric Oxide • neurotransmission • neuronal interconnections • nuclear membrane • nuclear pore • P • pinocytosis • proteasome • protein degradation • pumps • R • receptor proteins • receptor-mediated endocytosis • S • second messengers • signaling gradients • signal transduction • spindle • structure • T • transport • two-component systems • U • ubiquitin • V • vacuole • vesicle •
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.